17+ Calculate The Molar Heat Of Solution For Magnesium Sulfate
The heat capacity of Lithium Hydroxide is 2071 JgK. 43 g of tartaric acid C 4.

Chemistry Z Ibrahim Barro Id 5cf5838b4873f
It helps to correct the excess amount of acidity.
. Web Calculate your essay price. Compared with freshwater seawater prolongs the. A solution is 17 mL ethanol in 48 mL of solution.
Web How many moles of magnesium sulfate are in each box. The effect of seawater on properties and hydrates of the magnesium potassium phosphate MKP cement pastes with MgPO 4 molar ratio of 8 and low wc ratio of 015 were investigated in this study. In this example we are calculating the empirical formula for mass composition.
Web Calculate the molar mass of calcium nitrate. What is the percent volume of ethanol in this solution. Web Calcium sulfate antibiotic beads eg Osteoset Resorbable Mini-Bead no specific code.
Use the chemical formula for magnesium sulfate MgSO 4. O2 2 Mg 2 MgOIf 4 moles of magnesium totally reacted with more than enough O2 how many moles of MgO would be expected to form. Web Quantification of solubility.
75 g of potassium hydrogen phosphate in 250 mL of solution. Depending on context the term may or may not include ions which satisfy this criterion. Calculate the heat in J.
The movement in which the baby takes the nipple and areola properly into the mouth to begin breastfeeding. In quantum physics organic chemistry and biochemistry the distinction from ions is dropped and molecule is often used when referring to polyatomic ions. What is the mass percentage of iron sulfate in this solution.
Note that oxygen O has the subscript 4 following it. Web Solution for A 200 mL solution of NaOH is neutralized with 142 mL of 0200 M HBr. The most common example is the molar volume of a gas at STP Standard Temperature and Pressure which is equal to 224 L for 1 mole of any ideal gas at a temperature equal to 27315 K and a pressure.
What is the concentration of the original NaOH solution. A 2500-mL sample of a 00100 M solution of KIO. 0707T Injections bone substitute material eg calcium phosphate into subchondral bone defect ie bone marrow lesion bone bruise stress injury microtrabecular fracture including imaging guidance and arthroscopic assistance for joint visualization.
The most common form of nylon Nylon-6 contains 6368 carbon 1238 nitrogen 980 hydrogen and. 128 g of sodium hydrogen sulfate in 400 mL of solution. Web They are typically named by stating the name of the anhydrous component followed by the Greek prefix specifying the number of moles of water present then the word hydrate example.
Web In chemistry and thermodynamics the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state with all substances in their standard statesThe standard pressure value p 10 5 Pa 100 kPa 1 bar is. Web The molar mass of this base is 2395 gmol. However with increasing molar mass the increase in boiling point of successive 1-chloroalkanes becomes smaller.
Any of the several ways of expressing concentration of solutions can be used such as the mass volume or amount in moles of the solute for a specific mass volume or mole amount of. Web Polymethylimino2-hydroxytrimethylenehydrochloride produced by reaction of 11 molar ratio of methylamine and epichlorohydrin so that a 31-percent aqueous solution at 25 degC has a Stokes viscosity range of 25-40 as determined by ASTM method D1545-76 Reapproved. It requires 3204 mL of Na2S2O3 solution to titrate the I3 ions present.
D There are more atoms in 1 mole of magnesium than in 1 mole of carbon. 128 g of sodium hydrogen sulfate in 400 mL of solution. 1981 Standard Test Method for Viscosity of Transparent Liquids by.
Web Example Two. Is reacted with an excess of KI. Write and balance the equation for the reaction of S2O32 with I3 in acidic solution.
Web The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. B The mass of 1 mole of magnesium is greater than the mass of 1 mole of carbon. To calculate the molarity of a solution you need to know the number of moles of solute and the total volume of the solution.
The boiling point of this base is 924 These are some weak bases lists Now lets discuss some common uses of Bases. What is the molarity of the Na2S2O3 solution. Web What is the molar concentration of a a 500 L solution that contains 0800 mol of magnesium acetate.
Web However dispersion force strength increases with an increasing number of electrons which is proportional to molar mass. Web A The mass of 1 mole of carbon is greater than the mass of 1 mole of magnesium. C The mass of 1 mole of carbon is the same as the mass of 1 mole of magnesium.
Web A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds. The solubility of a specific solute in a specific solvent is generally expressed as the concentration of a saturated solution of the two. Moles MgOH2 788g MgOH2 1 mol MgOH2583g 0135 mol Find the number of moles of HNO3by multiplying the volume of the nitric acid solution in liters by its molarity.
452 What volume of 0812 M HCl in milliliters is required to titrate 145 g of NaOH to the equivalence point. E none of the above. Emitting heat and forming oxygen-containing compounds is an example of an.
Web What is the molar concentration of each solution. Determine the molar mass of the acid. 43 g of tartaric acid C 4.
Web Last menstrual period LMP. Calculate the molarity of a KCl solution prepared by dissolving 0898 moles of KCl in. 75 g of potassium hydrogen phosphate in 250 mL of solution.
How many molecules of sulfur trioxide are in 780 grams. The reaction of carbonate ion with magnesium ion to form solid magnesium carbonate is an example of an oxidation reduction reaction. 114 g of barium chloride in 350 mL of solution.
This lets us find the most appropriate writer for any type of assignment. It is used as an antacid. The first day of last menstrual period the date that is used to calculate the 40 weeks of pregnancy and a womans due date.
Pages 550 words Approximate price. Web Molarity moles of solutelitres of solution For example a 025 molL NaOH solution contains 025 mol of sodium hydroxide in every litre of solution. The balanced chemical equation is.
Uses of Some Common Bases Magnesium Hydroxide. Web To determine the limiting reactant calculate the number of moles of MgOH2 from its mass using the molar mass as a conversion factor. Web What is the molar concentration of each solution.
Web Diatomic O2 can react with the element magnesium to form magnesium oxide MgO. Web Seawater can be used as an alternative to freshwater for preparing cement-based materials. 114 g of barium chloride in 350 mL of solution.
Our global writing staff includes experienced ENL ESL academic writers in a variety of disciplines. Web The assay was stopped by fixing and permeabilizing the cells with 20 μl per well of a solution containing 05 wv formalin Sigma-Aldrich 005 vv Triton X-100 Sigma-Aldrich 10 mM. Hence the boiling points of 1-chloroalkanes increase with increasing molar mass.

2022 Eastern Regional Meeting Journal Of Investigative Medicine

Solved Enthalpy Of Solution Magnesium Sulfate Mgso 23 Chegg Com
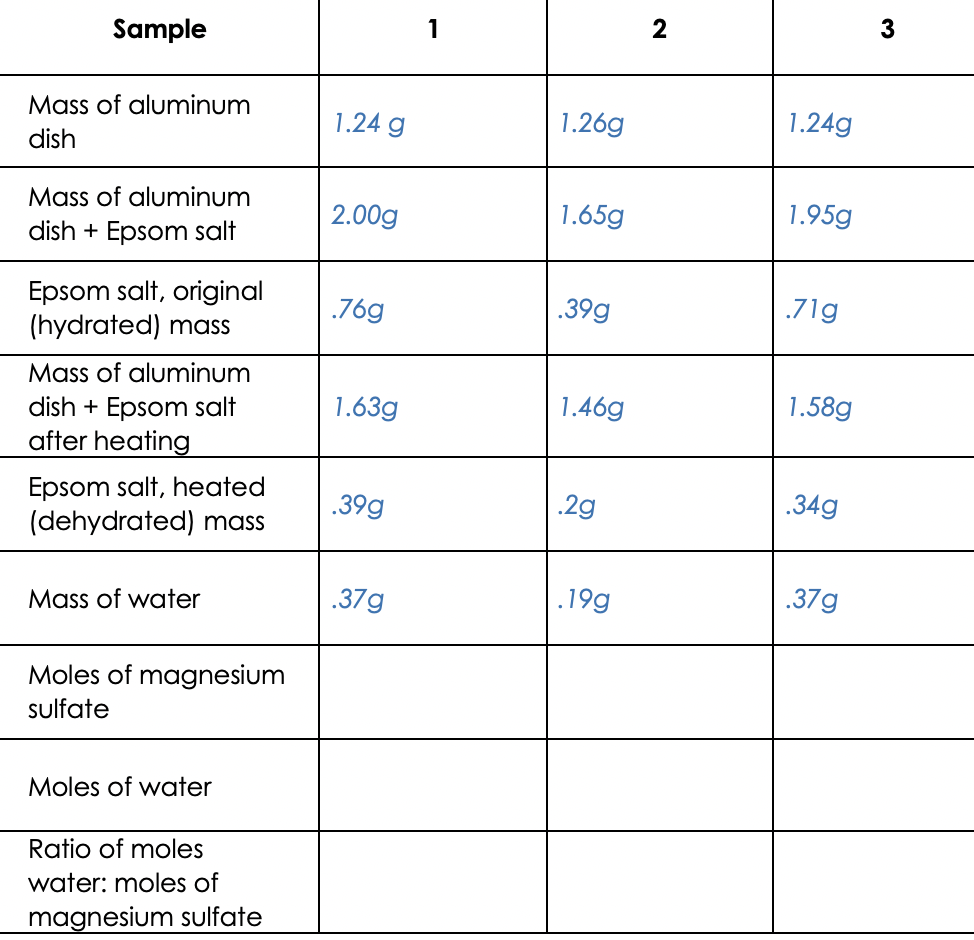
Solved 1 Calculate The Moles Of Magnesium Sulfate For Each Chegg Com

Enthalpy Change Of The Hydration Of Magnesium Sulphate A Level Science Marked By Teachers Com

Pearson Chemistry Y11 Pdf Chemical Reactions Periodic Table

What Is The Enthalpy Change Of Solution Of Mgso4 S In The Mixture Of 45g Of Water And 3 01g Of Mgso4 Quora

Solved Last Three Boxes Please It S Chemistry All The Chegg Com

Flow Assurance Solids In Oil And Gas Production Pdf Mole Unit Petroleum Reservoir

Doc Mg So4 Hydration Report Jessica Tan Academia Edu

Using Hess S Law To Calculate Enthalpy Change International Baccalaureate Chemistry Marked By Teachers Com

7 Calculate The Molar Heat Of Solution For Magnesium Sulfate Include The Course Hero
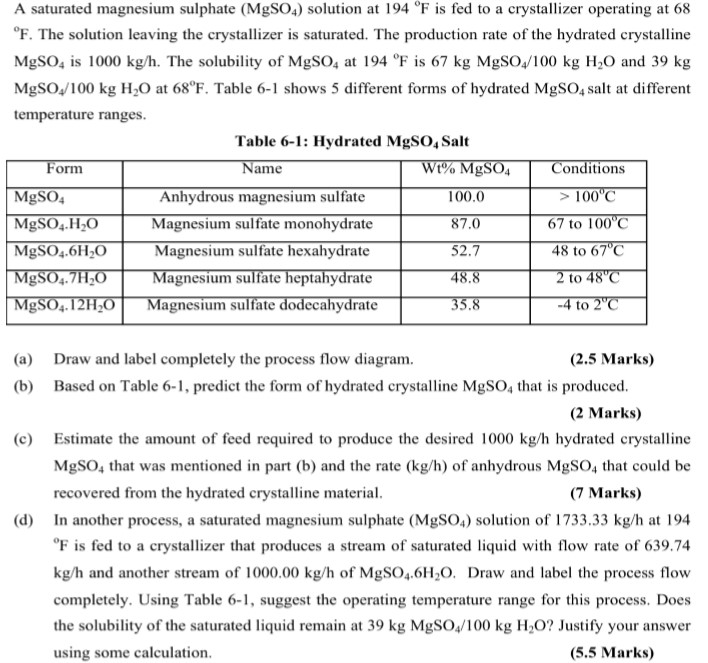
Solved A Saturated Magnesium Sulphate Mgso4 Solution At Chegg Com
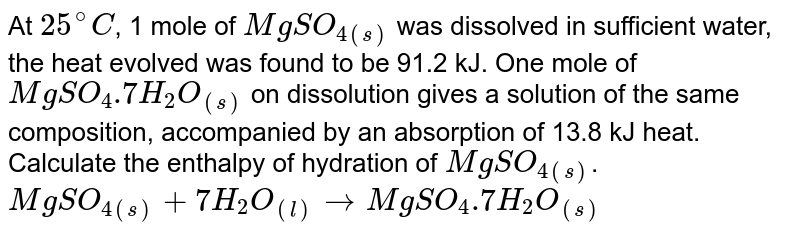
At 298 K The Heat Of Solution Of Mgso 4 S Is 91 21 Kj Mol 1 And That Of Mgso 4 7h 2 O S Is 13 81 Kj Mol 1 Calclate Heat Of Hydration Of Mgso 4 S I E Deltah For
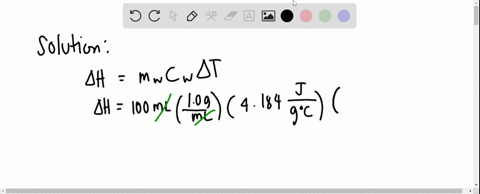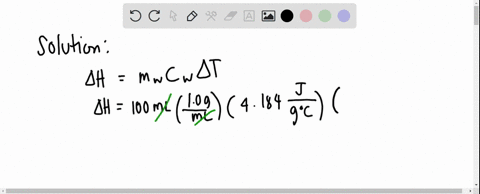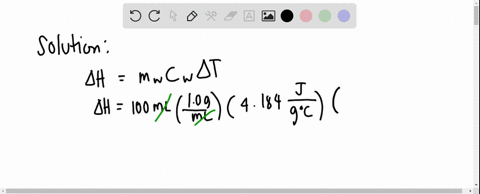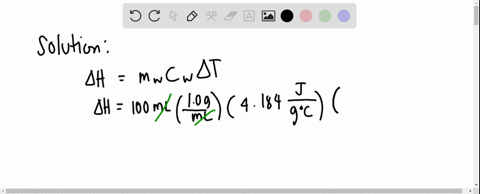
Solved 3 4 01 G Of Mgso4 Is Placed Into 80 0 Ml Of Water The Waters Temperature Increases By 6 45 C Calculate Hin Kk Mol For The Dissolution Of Mgso4 The Specific Heat

A2 Textbook Answers
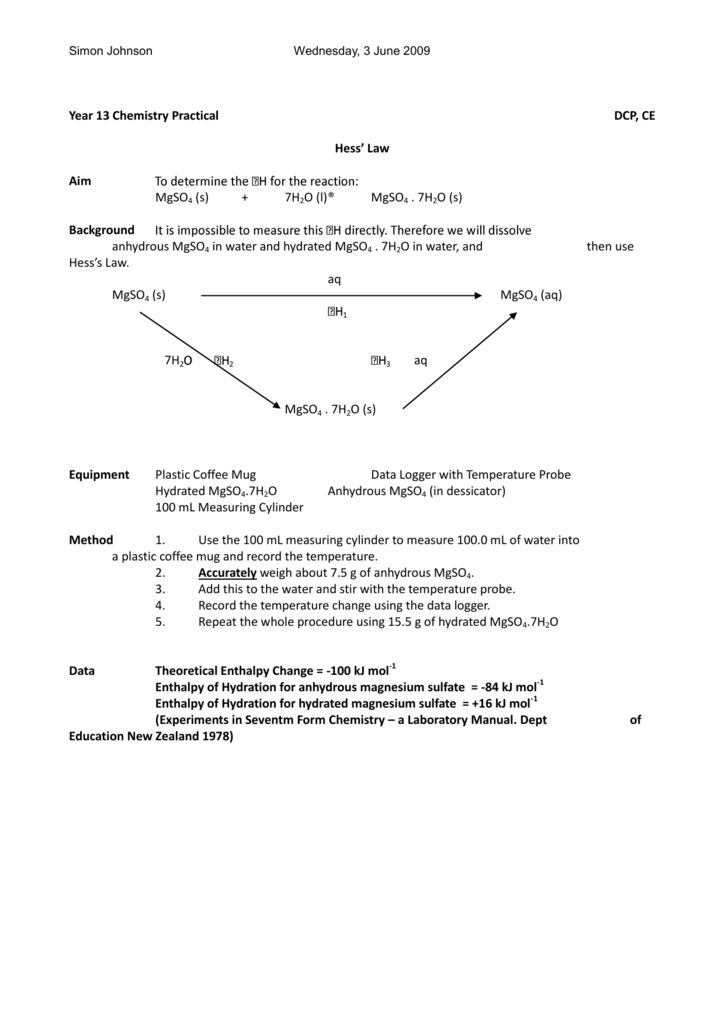
Hess S Law The Ib Guide

Solved 3 When 5 101 G Of Solid Magnesium Sulfate Chegg Com